Details of the Drug
General Information of Drug (ID: DMMVNZ0)
| Drug Name |
Masoprocol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NDGA; N 5023; TNP00263; Actinex (TN); Nordihydroguaiaretic acid (meso-form); (R*,S*)-4,4'-(2,3-Dimethylbutane-1,4-diyl)bispyrocatechol; 4-[(2S,3R)-4-(3,4-dihydroxyphenyl)-2,3-dimethylbutyl]benzene-1,2-diol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
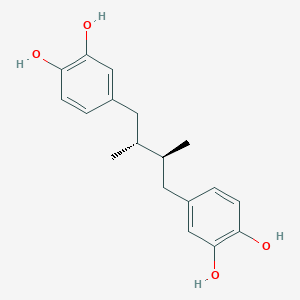 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 302.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Prostate cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2C82.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
